Massage Menu

Come visit us at our new location!
Your privacy is of utmost importance to us.
Schedule a free consultation today.


BOTOX® Cosmetic (onabotulinumtoxinA) Important Information
Indications BOTOX® Cosmetic (onabotulinumtoxinA) is indicated in adult patients for the temporary improvement in the appearance of: – moderate to severe glabellar lines associated with corrugator and/or procerus muscle activity – moderate to severe lateral canthal lines associated with orbicularis oculi activity – moderate to severe forehead lines associated with frontalis activity IMPORTANT SAFETY INFORMATION, INCLUDING BOXED WARNINGPlease see BOTOX® Cosmetic full Prescribing Information including Boxed Warning and Medication Guide.
ALLODERM SELECT™ Regenerative Tissue Matrix Indications and Important Safety Information
INDICATIONS ALLODERM SELECT™ Regenerative Tissue Matrix (ALLODERM SELECT™ RTM refers to both ALLODERM SELECT™ RTM and ALLODERM SELECT RESTORE™ RTM products) is intended to be used for repair or replacement of damaged or inadequate integumental tissue or for other homologous uses of human integument. This product is intended for single patient one-time use only. ALLODERM SELECT™ RTM is not indicated for use as a dural substitute or intended for use in veterinary applications. IMPORTANT SAFETY INFORMATION CONTRAINDICATIONS ALLODERM SELECT™ RTM should not be used in patients with a known sensitivity to any of the antibiotics listed on the package and/or Polysorbate 20. WARNINGS Processing of the tissue, laboratory testing, and careful donor screening minimize the risk of the donor tissue transmitting disease to the recipient patient. As with any processed donor tissue, ALLODERM SELECT™ RTM is not guaranteed to be free of all pathogens. No long-term studies have been conducted to evaluate the carcinogenic or mutagenic potential or reproductive impact of the clinical application of ALLODERM SELECT™ RTM. DO NOT re-sterilize ALLODERM SELECT™ RTM. DO NOT reuse once the tissue graft has been removed from the packaging and/or is in contact with a patient. Discard all open and unused portions of the product in accordance with standard medical practice and institutional protocols for disposal of human tissue. Once a package or container seal has been compromised, the tissue shall be either transplanted, if appropriate, or otherwise discarded. DO NOT use if the foil pouch is opened or damaged. DO NOT use if the seal is broken or compromised. DO NOT use if the temperature monitoring device does not display “OK.” DO NOT use after the expiration date noted on the label. Transfer ALLODERM SELECT™ RTM from the foil pouch aseptically. DO NOT place the foil pouch in the sterile field. PRECAUTIONS Poor general medical condition or any pathology that would limit the blood supply and compromise healing should be considered when selecting patients for implanting ALLODERM SELECT™ RTM as such conditions may compromise successful clinical outcome. Whenever clinical circumstances require implantation in a site that is contaminated or infected, appropriate local and/or systemic anti-infective measures should be taken. ALLODERM SELECT™ RTM has a distinct basement membrane (upper) and dermal surface (lower). When applied as an implant, it is recommended that the dermal side be placed against the most vascular tissue. Soak the tissue for a minimum of 2 minutes using a sterile basin and room temperature sterile saline or room temperature sterile lactated Ringer’s solution to cover the tissue. If any hair is visible, remove using aseptic technique before implantation. ALLODERM SELECT™ RTM should be hydrated and moist when the package is opened. DO NOT use if this product is dry. Use of this product is limited to specific health professionals (e.g., physicians, dentists, and/or podiatrists). Certain considerations should be made to reduce the risk of adverse events when performing surgical procedures using a tissue graft. Please see the Instructions for Use (IFU) for more information on patient/product selection and surgical procedures involving tissue implantation before using ALLODERM SELECT™ RTM. ADVERSE EVENTS The most commonly reported adverse events associated with the implant of a tissue graft include, but are not limited to the following: wound or systemic infection; seroma; dehiscence; hypersensitive, allergic or other immune response; and sloughing or failure of the graft. ALLODERM SELECT™ RTM is available by prescription only. For more information, please see the Instructions for Use (IFU) for ALLODERM SELECT™ RTM.To report an adverse reaction, please call Allergan at 1.800.433.8871.
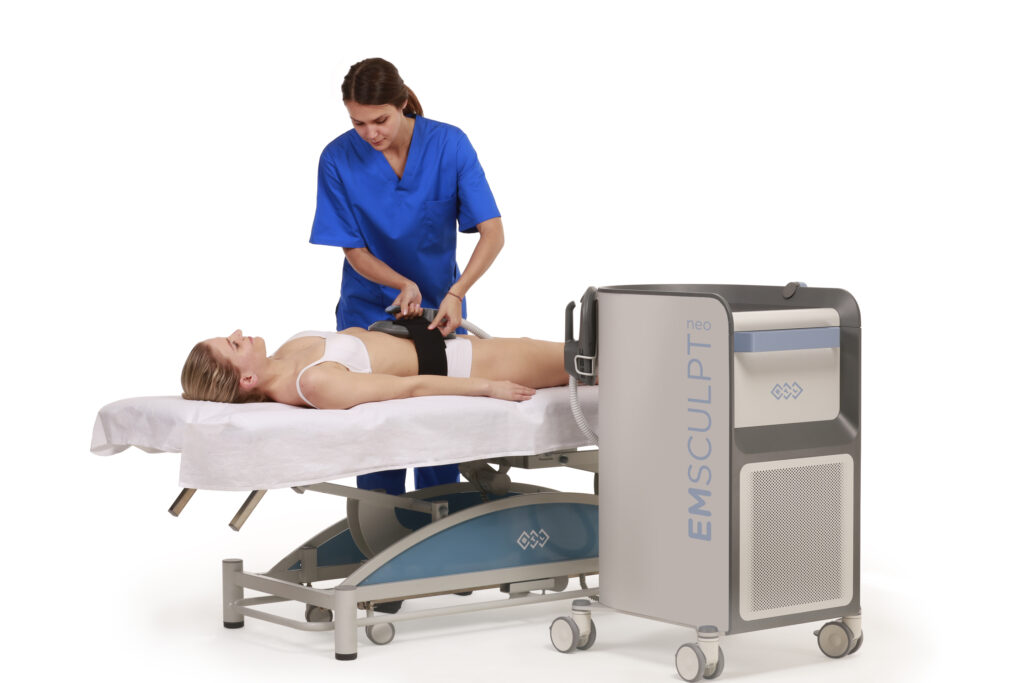
CoolSculpting® Treatment Important Information
The CoolSculpting® procedure is FDA-cleared for the treatment of visible fat bulges in the abdomen, flank, thigh, submental and submandibular areas, along with bra fat, back fat, underneath the buttocks (also known as the banana roll), and the upper arm. It is also FDA-cleared to affect the appearance of lax tissue with submental area treatments.During the procedure the patient may experience pinching, numbness, or discomfort. Following the procedure, typical side effects include temporary numbness, redness, swelling, bruising, firmness, tingling, stinging, and pain. Rare side effects may also occur. The CoolSculpting® procedure is not for everyone. Patients should not have the CoolSculpting® procedure if they suffer from cryoglobulinemia, cold agglutinin disease, or paroxysmal cold hemoglobinuria. The CoolSculpting® procedure is not a treatment for obesity. As with any medical procedure, a consultation should be done by a licensed physician to determine if the patient is a candidate for treatment. Consult the CoolSculpting® system user manual for a complete list of contraindications and potential side effects. Treatment applications that deviate from the guidelines are not recommended.
JUVÉDERM® Collection of Fillers Important Information
INDICATIONS JUVÉDERM VOLUMA® XC injectable gel is indicated for deep (subcutaneous and/or supraperiosteal) injection for cheek augmentation to correct age-related volume deficit in the mid-face in adults over the age of 21. JUVÉDERM® Ultra XC and JUVÉDERM® Ultra Plus XC injectable gels are indicated for injection into the mid-to-deep dermis for correction of moderate to severe facial wrinkles and folds (such as nasolabial folds). JUVÉDERM VOLLURE™ XC injectable gel is indicated for injection into the mid-to-deep dermis for correction of moderate to severe facial wrinkles and folds (such as nasolabial folds) in adults over the age of 21. JUVÉDERM® Ultra XC injectable gel is indicated for injection into the lips and perioral area for lip augmentation in adults over the age of 21. JUVÉDERM VOLBELLA® XC injectable gel is indicated for injection into the lips for lip augmentation and for correction of perioral rhytids in adults over the age of 21. IMPORTANT SAFETY INFORMATION CONTRAINDICATIONS These products should not be used in patients who have severe allergies, marked by a history of anaphylaxis or history or presence of multiple severe allergies, and should not be used in patients with a history of allergies to Gram-positive bacterial proteins or lidocaine contained in these products. WARNINGSProducts in the JUVÉDERM® Collection are available by prescription only.
KYBELLA® (deoxycholic acid) injection 10 mg/mL Important Information
INDICATION KYBELLA® (deoxycholic acid) injection is indicated for improvement in the appearance of moderate to severe convexity or fullness associated with submental fat in adults. The safe and effective use of KYBELLA® for the treatment of subcutaneous fat outside the submental region has not been established and is not recommended. IMPORTANT SAFETY INFORMATION CONTRAINDICATIONS KYBELLA® is contraindicated in the presence of infection at the injection sites. WARNINGS AND PRECAUTIONS Marginal Mandibular Nerve Injury Cases of marginal mandibular nerve injury, manifested as an asymmetric smile or facial muscle weakness, were reported in 4% of subjects in the clinical trials; all cases resolved spontaneously (range 1-298 days, median 44 days). KYBELLA® should not be injected into or in close proximity to the marginal mandibular branch of the facial nerve. Dysphagia Dysphagia occurred in 2% of subjects in the clinical trials in the setting of administration-site reactions, eg, pain, swelling, and induration of the submental area; all cases of dysphagia resolved spontaneously (range 1-81 days, median 3 days). Avoid use of KYBELLA® in patients with current or prior history of dysphagia as treatment may exacerbate the condition. Injection-Site Hematoma/Bruising In clinical trials, 72% of subjects treated with KYBELLA® experienced hematoma/bruising. KYBELLA® should be used with caution in patients with bleeding abnormalities or who are currently being treated with antiplatelet or anticoagulant therapy as excessive bleeding or bruising in the treatment area may occur. Risk of Injecting Into or in Proximity to Vulnerable Anatomic Structures To avoid the potential of tissue damage, KYBELLA® should not be injected into or in close proximity (1 cm-1.5 cm) to salivary glands, lymph nodes, and muscles. Injection Site Alopecia Cases of injection site alopecia have been reported with administration of KYBELLA®. Onset and duration may vary among individuals and may persist. Consider withholding subsequent treatments until resolution. Injection Site Ulceration and Necrosis Injections that are too superficial into the dermis may result in skin ulceration and necrosis. Cases of injection site ulceration and necrosis have been reported with administration of KYBELLA®. Do not administer KYBELLA® into affected area until complete resolution. ADVERSE REACTIONS The most commonly reported adverse reactions in the pivotal clinical trials were: injection site edema/swelling, hematoma/bruising, pain, numbness, erythema, and induration.Please see KYBELLA® full Prescribing Information.
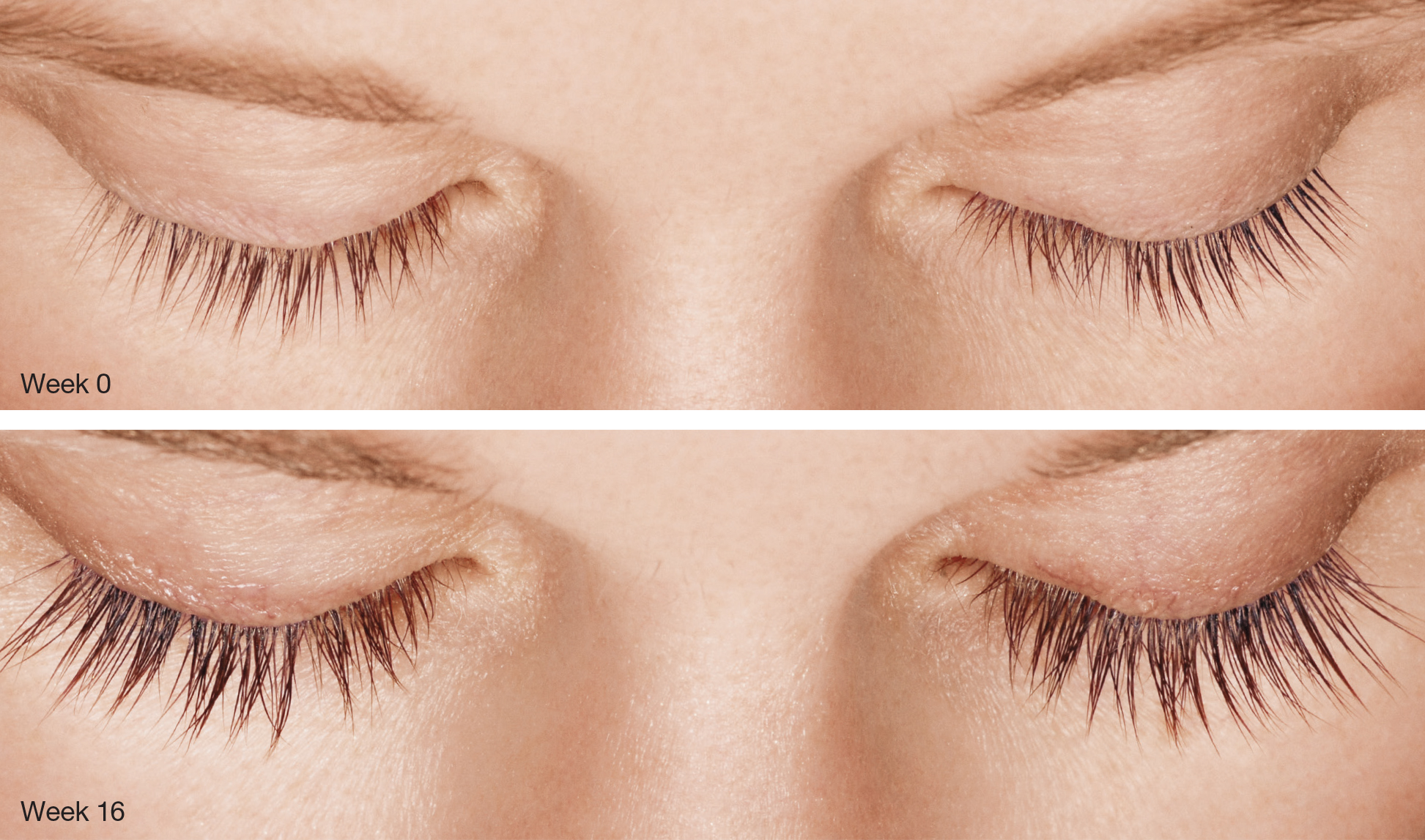
LATISSE® (bimatoprost ophthalmic solution) 0.03% Important Information
Indication LATISSE® (bimatoprost ophthalmic solution) 0.03% is indicated to treat hypotrichosis of the eyelashes by increasing their growth, including length, thickness, and darkness. Important Safety Information Contraindications: LATISSE® is contraindicated in patients with hypersensitivity to bimatoprost or to any of the ingredients. Warnings and Precautions: In patients using LUMIGAN® (bimatoprost ophthalmic solution) or other prostaglandin analogs for the treatment of elevated intraocular pressure (IOP), the concomitant use of LATISSE® may interfere with the desired reduction in IOP. Patients using prostaglandin analogs including LUMIGAN® for IOP reduction should only use LATISSE® after consulting with their physician and should be monitored for changes to their intraocular pressure. Increased iris pigmentation has occurred when bimatoprost solution was administered. Patients should be advised about the potential for increased brown iris pigmentation, which is likely to be permanent. Bimatoprost has been reported to cause pigment changes (darkening) to periorbital pigmented tissues and eyelashes. The pigmentation is expected to increase as long as bimatoprost is administered, but has been reported to be reversible upon discontinuation of bimatoprost in most patients. There is the potential for hair growth to occur in areas where LATISSE® solution comes in repeated contact with skin surfaces. Apply LATISSE® only to the skin of the upper eyelid margin at the base of the eyelashes. LATISSE® solution should be used with caution in patients with active intraocular inflammation (eg, uveitis) because the inflammation may be exacerbated. LATISSE® should be used with caution in aphakic patients, in pseudophakic patients with a torn posterior lens capsule, or in patients with known risk factors for macular edema. Adverse Reactions: The most frequently reported adverse events were eye pruritus, conjunctival hyperemia, skin hyperpigmentation, ocular irritation, dry eye symptoms, and periorbital erythema. These reactions occurred in less than 4% of patients. Postmarketing Experience: The following adverse reactions have been identified during postapproval use of LATISSE®: dry skin of the eyelid and/or periocular area, eye swelling, eyelid edema, hypersensitivity (local allergic reactions), lacrimation increased, madarosis and trichorrhexis (temporary loss of a few eyelashes to loss of sections of eyelashes, and temporary eyelash breakage, respectively), periorbital and lid changes associated with a deepening of the eyelid sulcus, rash (including macular and erythematous), skin discoloration (periorbital), and vision blurred.Please see LATISSE® full Prescribing Information.
Natrelle® Breast Implants Important Information
INDICATIONS Natrelle® Breast Implants are indicated for women for the following:Natrelle® Breast Implants are available by prescription only.
REVOLVE™ Advanced Adipose System Indications and Important Safety Information
INDICATIONS The REVOLVE™ Advanced Adipose System (REVOLVE™ System) is used for aspiration, harvesting, filtering, and transferring of autologous adipose tissue for aesthetic body contouring. This system should be used with a legally marketed vacuum or aspirator apparatus as a source of suction. If harvested fat is to be re-implanted, the harvested fat is only to be used without any additional manipulation. REVOLVE™ System is intended for use in the following surgical specialties when the aspiration of soft tissue is desired: plastic and reconstructive surgery, gastrointestinal and affiliated organ surgery, urological surgery, general surgery, orthopedic surgery, gynecological surgery, thoracic surgery, and laparoscopic surgery. IMPORTANT SAFETY INFORMATION CONTRAINDICATIONS Contraindications to autologous fat transfer include the presence of any disease processes that adversely affect wound healing, and poor overall health status of the individual. WARNINGS REVOLVE™ System must be used within the same surgical procedure. Reuse of this device in the same patient in a subsequent surgical procedure, or for more than one patient, may result in infection and/or transmission of communicable diseases. Do not use the product if sterile packaging is damaged. This device will not, in and of itself, produce significant weight reduction. This device should be used with extreme caution in patients with chronic medical conditions such as diabetes, heart, lung, or circulatory system disease or obesity. The volume of blood loss and endogenous body fluid loss may adversely affect intra and/or postoperative hemodynamic stability and patient safety. The capability of providing adequate, timely replacement is essential for patient safety. PRECAUTIONS REVOLVE™ System is designed to remove localized deposits of excess fat through small incision and subsequently transfer the tissue back to the patient. Use of this device is limited to those physicians who, by means of formal professional training or sanctioned continuing medical education (including supervised operative experience), have attained proficiency in suction lipoplasty and tissue transfer. Results of this procedure will vary depending upon patient age, surgical site, and experience of the physician. Results of this procedure may or may not be permanent. The amount of fat removed should be limited to that necessary to achieve a desired cosmetic effect. Filling the device with adipose tissue over the maximum fill volume line can lead to occlusion of the mesh resulting in mesh tear. ADVERSE EFFECTS Some common adverse effects associated with autologous fat transfer are asymmetry, over- and/or under-correction of the treatment site, tissue lumps, bleeding, and scarring. Potential adverse effects associated with REVOLVE™ System include fat necrosis, cyst formation, infection, chronic foreign body response, allergic reaction, and inflammation. REVOLVE™ System is available by prescription only. For more information, please see the Instructions for Use (IFU) and User Manual for REVOLVE™ System.To report an adverse reaction, please call Allergan at 1.800.367.5737.




 Full Arm$40.00 Half Arm$20.00 Full Leg$80.00 Half Leg$40.00 Full Back$80.00 Half Back$40.00 Full Stomach$60.00 Half Stomach$30.00 Chest$40.00 Neck$15.00 Face$30.00 Chin$10.00 Ears$10.00 Lip$10.00 Nose$10.00 Sideburns$15.00 Hand$15.00 Under Arms$20.00 Bikini$45.00 Brazilian Bikini$75.00
Full Arm$40.00 Half Arm$20.00 Full Leg$80.00 Half Leg$40.00 Full Back$80.00 Half Back$40.00 Full Stomach$60.00 Half Stomach$30.00 Chest$40.00 Neck$15.00 Face$30.00 Chin$10.00 Ears$10.00 Lip$10.00 Nose$10.00 Sideburns$15.00 Hand$15.00 Under Arms$20.00 Bikini$45.00 Brazilian Bikini$75.00 HORMONE REPLACEMENTConsultation ERECTILE DYSFUNCTIONConsultation FEMALE SEXUAL DYSFUNCTIONConsultation
HORMONE REPLACEMENTConsultation ERECTILE DYSFUNCTIONConsultation FEMALE SEXUAL DYSFUNCTIONConsultation